This is a guest article written by Matthew Loxton, a Principal Healthcare Analyst and Professional MAXQDA Trainer.
With the inclusion of the Logbook function, MAXQDA can be used effectively to document and control research planning, administration, and execution (PAE) from within a MAXQDA project.

In our MAXQDA International Conference 2019 poster “‘Patient Voice’ Uncovering The Voices of Radiology Patients” (below), we described a specific research project, in which MAXQDA played this central integrating role.
PAE Information
One of the challenges in research projects is to provide historical PAE information that will help control the study, enable others to learn from risks, issues, and opportunities – and to aid in the replication of a study.
Documenting the entire end-to-end lifespan of a research project in MAXQDA not only provides this information for managing the project better but also forms the basis for constructive self-analysis. Data about the project itself can be analyzed within MAXQDA to inform process improvement of research planning and execution methods.
MAXQDA Advantages
The advantages of using MAXQDA as a PAE hub include improved efficiency and risk agility due to having a single workspace, enhancing process improvement, and improved study replicability.
- Single Workspace: Team members all have the PAE information in the MAXQDA project where they will be doing transcription and analysis. Thus, no need for secondary storage, file access, or risk of people working on different versions of PAE documents. This results in fewer administrative steps, and lower risk of confusion.
- Process Improvement: Project Risks, Issues, and Opportunities documented in MAXQDA are available to be analyzed using MAXQDA. These data can be used to develop Lessons Learned, identify improvement opportunities, and to develop evidence-based quality improvement interventions. Process improvement reports can be created directly in MAXQDA.
- Increased Replicability: Because the project steps and elements are kept within the MAXQDA project, there is a timestamped trail of evidence of the project logic and decisions made, parameters used for searches and autocoding, and criteria used for MAXQDA’s MAXMaps and MAXDictio features. Statistical functions and their parameters are also documented within the project. As a result, replication is easier.
Our “Patient Voice” Study
Recording our steps pre-approval
In the Patient Voice study (click the image of our poster below to open a full-screen PDF), we started documenting in the MAXQDA project Logbook long before we even had an approved research study. Had the project not been approved, the reasons would have been logged for future reference.

“Patient Voice”: Uncovering The Voices of Radiology Patients
by M. H. Loxton / E. Okoli / Whitney, Bradley, & Brown Inc
We created the MAXQDA project and used the Logbook function to outline the research need, goals, and scope, and document discussions of research ideas with internal stakeholders. This process allowed us to leave a history of the project creation that all researchers can access within the MAXQDA project. These notes were frequently used by the team to refresh their memory on the study scope and purpose, as well as to keep the project within the parameters.
As can be seen in Figure 1, there are two document sets in MAXQDA’s “Document System” window: the interview data, and the PAE documents.
- The interview data set (collapsed in the figure) included all interview recordings and transcripts, notes, and follow-up questions and answers.
- The PAE document set included several documents related to how the project was to be run, its scope, and all perceived risks and their mitigation plans. The PAE document set also included the project communication plan that described how communication would be carried out and with whom.
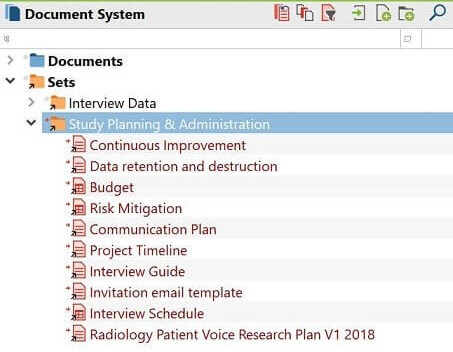
Figure 1: Document sets with interview data and PAE documents in MAXQDA
These documents could be seen by everyone in the team and were used to explore opportunities to improve the project.
Post-announcement, pre-launch
Once the Patient Voice research project was declared internally, this event was documented in the Logbook. The research plan was outlined in the Logbook, and the plan MS Word document was imported as part of a Planning & Administration document set.
The internal discussions regarding funding alternatives, publication options, and scope and timing constraints were recorded in the Logbook as events, and any associated minutes or documents were imported and placed in the Planning & Administration document set.
Launching the study
Once the Patient Voice study was launched, this event was recorded in the Logbook. We also captured the details of launching a project site in the Logbook. For example, it specified how we set a $1,000 target for covering administrative costs such as postage and $25 rewards for interview participants, and detailed the process used to recruit and schedule interview participants.
We recorded how we planned to invite participants, and how we announced the study and funding request on social media, including Twitter, LinkedIn, and online patient community portals. This level of detail of PAE within the MAXQDA project allowed the team to easily refer to why decisions were made, and provides a historical reference for future projects.
Twitter Data Analysis with MAXQDA
Recording administrative steps
The daily administration of interview scheduling, handling no-shows or postponement requests, and dispatching the gift cards was recoded in Logbook, and any associated documents such as the email templates and interview schedules were imported and placed into the document set.
This also made reference and reuse convenient for the research team, and the Logbook recorded the changes and adaptations made to the templates over time for future reference.
Completing the study
The logbook also recorded the steps to shut down the project at completion, and critical events such as deletions and erasure of records to achieve the level of anonymity required.
Figure 2 shows the early use of the Logbook to record project progress and planning.

MAXQDA-Logbook showing the project progress and planning
Conclusion
Two features of MAXQDA make centralization of planning, administration, and execution within the MAXQDA project a practical reality – the Logbook and Document Sets.
The use of MAXQDA to log our planning, administration, and execution of the study provided a clear sequence of events that helped us to perform more efficiently by creating a single central place for reference materials within the project itself. This reduced the risk of members working in different copies of documents and plans, and built a researchable record of how the study was done.
A secondary but equally important benefit was having all the associated documentation inside MAXQDA for conducting process improvement research. From this, we could start building a “Lessons Learned” catalogue to help us improve our methods and processes in the future.
About the Author
Matthew Loxton is a Principal Analyst at Whitney, Bradley, and Brown Inc. focused on healthcare improvement, serves on the board of directors of the Blue Faery Liver Cancer Association, and holds a master’s degree in KM from the University of Canberra. Matthew is the founder of the Monitoring & Evaluation, Quality Assurance, and Process Improvement (MEQAPI) organization, and regularly blogs for Physician’s Weekly. Matthew is active on social media related to healthcare improvement and hosts the weekly #MEQAPI chat. You can also read other guest posts by Mathew Loxton on Mixed Methods Research here in the MAXQDA Research Blog.

 Matthew Loxton is a Principal Analyst at Whitney, Bradley, and Brown Inc. focused on healthcare improvement, serves on the board of directors of the
Matthew Loxton is a Principal Analyst at Whitney, Bradley, and Brown Inc. focused on healthcare improvement, serves on the board of directors of the